How to dry aerogels without cracks.
Use supercritical fluid to dry your aerogels.
Removing the aerogels from the solvent bath for common use is fraught with problems. Because the structure is so fine, normal drying at atmosphere may collapse the network into dust. This is from the normal capillary pressure at the liquid/vapor interface on the inside of the pore.
Like gases, supercritical fluids exhibit no surface tension. It is this property that allows for the aerogel to be dried without destruction.
Several methods can be used to dry aerogels:
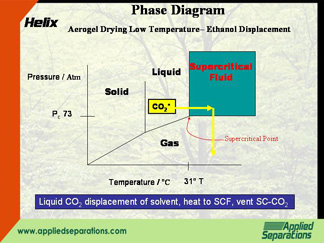
- Liquid CO2 displacement of an organic solvent (usually ethanol) with subsequent supercritical CO2 venting (Hunt process*)
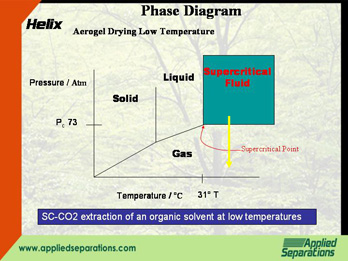
- Supercritical CO2 extraction of an organic solvent at low temperatures
- High-temperature conversion of a liquid organic solvent to the supercritical state with subsequent venting
* Dr. Arlon Hunt at Lawrence Berkeley National Laboratory

Aerogels are highly porous materials with large internal surface area and large pore volumes. Their densities can be as low as 3 kg/m3 and have porosities as high as 99.9%. This makes them excellent thermal, electrical, and acoustic insulators. A gel is normally produced in a liquid requiring specialty drying.
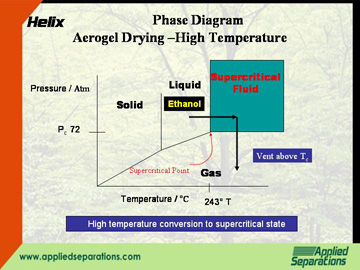
High-temperature conversion of a liquid organic solvent to the supercritical state with subsequent venting
With this method, the liquid organic solvent (e.g. ethanol) is heated and pressurized above its critical point (243°C, 74 BAR). The ethanol is then released to atmosphere while maintaining an elevated temperature. If this method is used, appropriate measures need to be taken to ensure safety in dealing with flammable compounds.
Liquid CO2 displacement of an organic solvent with subsequent supercritical CO2 venting
Developed in the 1980s, in this method, the ethanol is first displaced with liquid CO2. The CO2 is pressurized above the supercritical point (73 atm, 31°C). The supercritical CO2 is then depressurized keeping the temperature above 31°C.
Supercritical CO2 extraction of an organic solvent at low temperature
Similar to the liquid displacement method, the ethanol is this time-displaced and extracted with supercritical CO2. The supercritical CO2 is then depressurized keeping the temperature above 31°C.