Extract Natural Products with Supercritical Fluids
The term natural products has become the "catch-all" for any compound that has been produced by a living being, e.g. plant, animal, algae. The extracted compounds are used in or are themselves, foods, medicinals, pigments, and fragrances. The process for many years was to extract from the matrix material by solvents: aqueous and petroleum-based. The first large-scale use of supercritical fluids in extracting natural products was the decaffeination of coffee in 1979 and since then thousands of compounds have been extracted commercially.


 Why the interest in using supercritical fluids (i.e. CO2)?
Why the interest in using supercritical fluids (i.e. CO2)?
No Solvent residue. No health hazards. Maintains a "natural" state.
Supercritical CO2 extracts the various natural compounds more efficiently than petroleum-based solvents, but upon returning to an ambient state, the CO2 becomes a gas, leaving no residue.
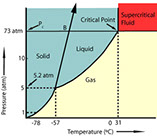
Mild Extraction Conditions – 31°C temperature
With temperatures less than body temperature (37°C), little thermal degradation of sensitive compounds occurs. (See phase diagram.)
Fractionation - easy using only CO2: CO2 is a “tunable solvent”
Load the feedstock into an extraction vessel only once and then by only changing the pressure of the supercritical CO2, you can make it have the solubility characteristics of a myriad of petroleum-based solvents. You don’t need to add or change solvents.